Thalassemia, myelodysplastic syndrome, hereditary hemochromatosis, and other iron metabolic disorders can lead to iron overload in the body. This condition is characterized by elevated levels of serum iron in the blood, transferrin saturation and serum ferritin, and often by the presence of iron not bound to transferrin (NTBI).
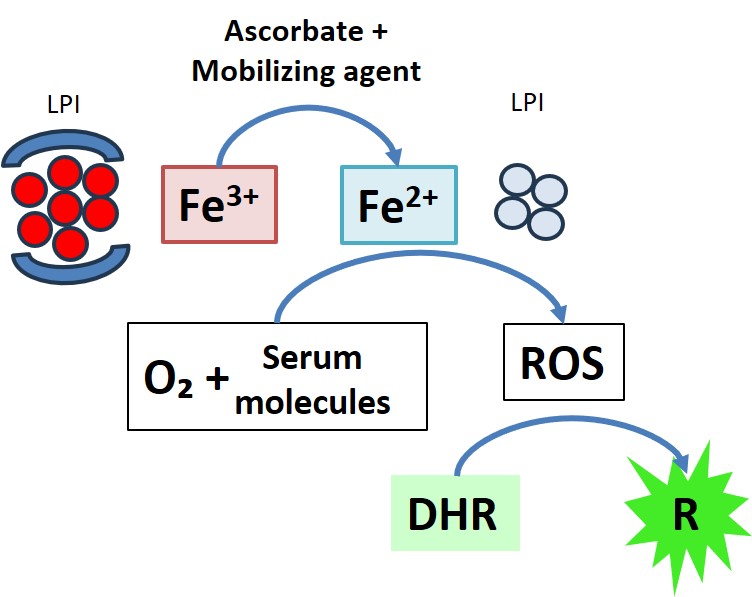
In many patients with iron overload, part of the NTBI can generate reactive oxygen species (ROS) in the blood and in the affected cells. This can cause damage to sensitive organs such as the liver, the pancreas, and especially the heart.
We offer an informative method to measure iron levels in serum/plasma. This assay detects Non-Transferrin Bound Iron (NTBI) and measures the specific redox activity of iron in plasma using fluorescence technology. The assay provides a measure of potentially toxic iron in plasma that is active in redox (labile) because it is not bound to transferrin. This innovative tool allows monitoring of patients receiving regular transfusions and chelation therapy. The clinical use of these biomarkers helps assess whether there is iron overload in the blood and determine the effectiveness of chelation treatments.
Doubts / Errors
If you encounter any error in the buying process, please let us know:

